Comparative Study of NCA vs NCM Cathode Materials: Efficiency, Stability, and Safety
Lithium-ion batteries have become the backbone of modern energy storage, powering everything from portable electronics to electric vehicles. Central to their performance are the cathode materials, which largely determine such key characteristics as energy density, cycle life, and safety. Currently, two of the most wide-spread cathodes in use are Lithium Nickel Cobalt Aluminum Oxide, or NCA, and Lithium Nickel Cobalt Manganese Oxide, or NCM. While both materials are nickel-rich and share many characteristics, there are important differences in their composition, performance, and safety profiles that make each suited to specific applications.
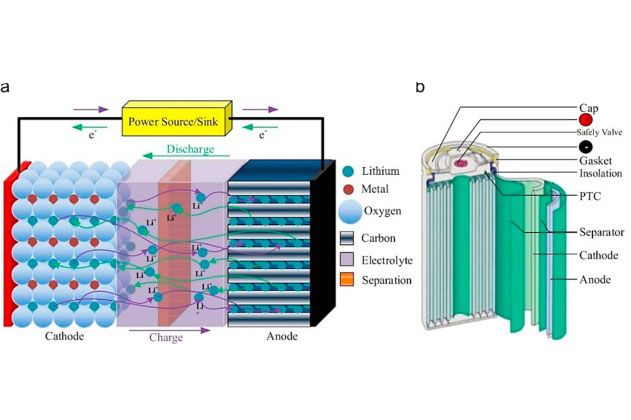 [1]
[1]
Composition and Structural Characteristics
NCA represents, in general, a chemical formula like LiNi₀.₈Co₀.₁₅Al₀.₀₅O₂, in which aluminum is incorporated to enhance structural stability and minimize the degradation in cycling. The high percentage of nickel provides substantial capacity; at the same time, the presence of cobalt serves to stabilize the crystal lattice, while aluminum reduces unwanted side reactions. NCA cathodes, because of their specific capacities of 200–220 mAh/g, are mainly applied to high-energy-density applications such as Tesla EV batteries.
NCM, or NMC, has several variants, such as NCM 111, NCM 532, NCM 622, and NCM 811, each representing the ratio of nickel, cobalt, and manganese. For instance, NCM 811 is made up of 80% nickel, 10% cobalt, and 10% manganese. Manganese acts to improve thermal stability and safety, cobalt improves structural stability, and nickel acts to increase capacity. NCM cathodes generally offer 160–200 mAh/g, somewhat lower compared to NCA, but with better thermal tolerance.
X-ray diffraction analysis revealed that both NCA and NCM had a similar structure of layered α-NaFeO₂, within which lithium ions could be intercalated and deintercalated efficiently. The main difference between the two structures is in the doped elements: aluminum in NCA and manganese in NCM.
Efficiency and Energy Density
The main characteristics evaluated when assessing battery efficiency are energy density and specific capacity.
• NCA Cathodes: High nickel content gives NCA cathodes a very high energy density, mostly above 260 Wh/kg in commercial EV batteries. These enable electric vehicles to drive for quite an extended period with a single full charge. In fact, for maximum range without increasing the size of their batteries, Tesla Model S and Model 3 vehicles use NCA cathodes in their battery packs.
• NCM Cathodes: Slightly lower energy density than NCA, typically in the region of 220–240 Wh/kg, depending on the nickel ratio.
However, the higher manganese content in NCM 622 or 811 improves cycle stability, which makes this chemistry particularly well-suited to applications where longevity is more important than maximum energy density. NCM cathodes are used by many manufacturers, including LG Chem and CATL, for both EV and stationary storage solutions.
In practical terms, NCA is preferred in applications needing compact, high-capacity batteries, whereas NCM offers a balance between energy density and cycling performance, making it versatile across multiple applications.
Stability and Cycle Life
The structural resilience of the cathode material during repeated lithium intercalation is an essential factor for battery life and long-term stability.
• NCA Stability: Aluminum improves the lattice stability of NCA, while the high nickel content increases the susceptibility to structural degradation at high temperatures or high voltages (>4.2 V). NCA batteries can normally maintain 80–85% capacity after 1000 cycles under regular EV conditions. However, it demands precise thermal management to avoid accelerated aging.
• NCM Stability: Manganese incorporation into NCM cathodes enhances thermal stability and reduces oxygen release from the lattice in conditions of overcharge or overheating. NCM 811, with its high nickel content, can approach NCA in capacity while retaining slightly better cycling stability, typically at 85–90% of capacity after 1000 cycles in controlled conditions.
Surface coatings and electrolyte additives can enhance the stability of these two important materials. For instance, Al₂O₃ or ZrO₂ coatings on NCA cathodes reduce transition metal dissolution, while LiPF₆-based electrolytes with additives help to mitigate structural degradation in both NCA and NCM.
Safety Considerations
Safety is one of the prime concerns of high-energy lithium-ion batteries. Nickel-rich cathodes have been commonly associated with thermal runaway, short-circuiting, and oxygen release.
• NCA Safety: High nickel content in NCA makes it more reactive at high temperatures, raising the risk of thermal runaway for overcharging and physical damage to batteries. Advanced BMS and precise thermal controls become necessary to use NCA safely in an EV.
• NCM Safety: Manganese in NCM acts as a stabilizing element, reducing exothermic reaction and generally providing better safety under abuse conditions. NCM cathodes are intrinsically slightly safer than NCA, especially when the application involves fluctuating temperatures or high current loads.
Safety concerns in practical applications can be realized when proper design is considered in both, including cell balancing, separators, and thermal management.
Applications and Market Trends
• NCA Cathodes: They are normally used in high-performance EVs, such as Tesla; high-energy portable devices; and aerospace applications because maximum energy density is important.
• NCM Cathodes: Utilized in electric vehicles, stationary storage, and consumer electronics, with an increasing NCM 811 adoption for electric vehicles striking a balance between performance, safety, and cost.
Market trends mark interest in the use of NCM 811 because of its lessened amount of cobalt, hence mitigating both financial and ethical concerns. Meanwhile, NCA maintains dominance in high-performance EVs where energy density is paramount.
Conclusion
NCA and NCM are the most critical cathode materials in lithium-ion batteries. While NCA has a big advantage in the energy density of the active material, making it much more ideal for long-range electrical vehicles or high-capacity, compact devices, it does demand careful thermal management. The balance between capacity, stability, and safety is ensured by NCM, with manganese improving thermal resilience and cycle life. For more information, please check Stanford Electronics.
Reference
[1] M.S. Hossain Lipu, M.A. Hannan, Aini Hussain, M.M. Hoque, Pin J. Ker, M.H.M. Saad, Afida Ayob, A review of state of health and remaining useful life estimation methods for lithium-ion battery in electric vehicles: Challenges and recommendations, Journal of Cleaner Production, Volume 205, 2018, Pages 115-133, ISSN 0959-6526.