Lithium Iron Phosphate (LFP) Powder: Properties, Synthesis, and Applications
Introduction
Lithium Iron Phosphate (LiFePO₄), also called LFP, is a very common cathode material in modern lithium-ion batteries. First reported in the late 1990s as thermally stable and safe compared with lithium cobalt oxide, LFP soon gained widespread use for applications where cycle life, stability, and safety are critical concerns. Its usage has been generalized from portable devices to electric vehicles (EVs) and energy storage systems of large-scale. The olivine-type composition of LFP, along with its environmental sustainability and economic merits, has made it the cornerstone in the transition towards sustainable energy.

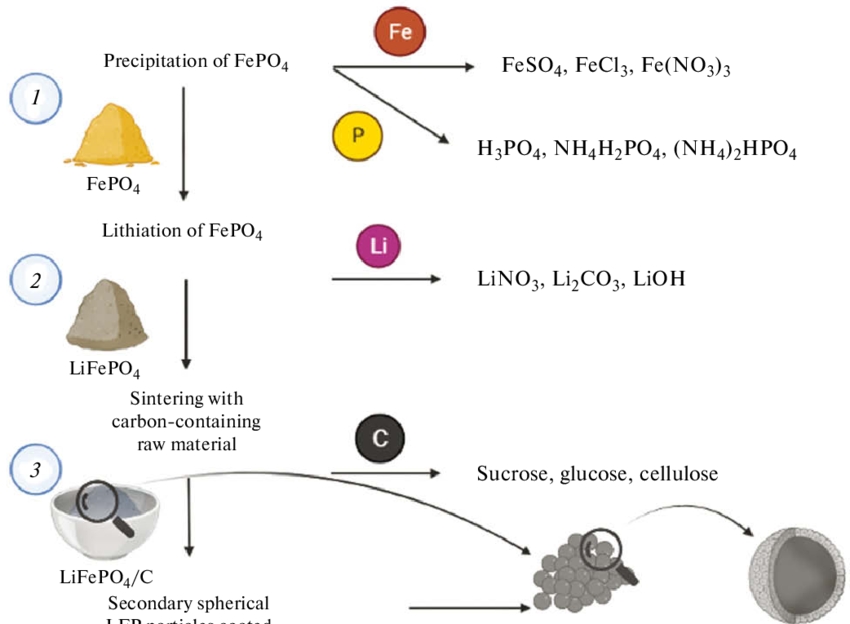 [1]
[1]
Chemical Composition and Crystal Structure
LFP has the composition LiFePO₄, which involves lithium, iron, and phosphate ions in an olivine-type crystal structure. It is characterized by FeO₆ octahedra linked to PO₄ tetrahedra, creating a robust host for lithium-ion intercalation and deintercalation. The phosphate group offers higher thermal stability through stabilization of the crystal lattice, thereby becoming less prone to oxygen release during overcharge or high-temperature operation.
The structure of olivine allows lithium ions to move along one-dimensional tunnels. While the arrangement offers less ionic conductivity than layered oxides, it facilitates greater structural stability with repeated charge–discharge cycles, giving increased battery life.
Key Properties of LFP Powder
Physical Properties
LFP powder is typically gray to black fine powder having particle size ranges from nanometers up to a few micrometers based on the synthesis method. Minimized particles maximize the surface area and minimize lithium-ion diffusion distances. Uniform morphology and well-controlled particle size distribution are required for homogeneous performance. The LFP density is around 3.6 g/cm³ and its stable structure prevents it from large expansion on cycling.
Electrochemical Properties
LFP's theoretical specific capacity is ca. 170 mAh/g, with a flat discharge voltage plateau at 3.2 V vs. Li/Li⁺. It has high cycle life, normally greater than 2000 charge–discharge cycles with little capacity loss. Its intrinsic electronic conductivity is quite low, but synthesis and modification methods overcome this limitation. LFP also has excellent thermal stability and does not suffer from thermal runaway under regular operating conditions, making it one of the safest cathode materials.
Mechanical and Thermal Properties
Thermogravimetric analysis shows that LFP is stable to approximately 500 °C before decomposition becomes significant. The phosphate structure inhibits oxygen release even under mechanical abuse or overcharging. Such thermal stability is one of the major causes of employment in stringent safety applications.
Case Study: Lithium Iron Phosphate Powder for Batteries
Synthesis Methods of LFP Powder
Solid-State Reaction
This conventional method relies on the blending of lithium, iron, and phosphate precursors—typically carbonates, oxides, and phosphates—followed by subsequent high-temperature calcination. While cheap, it tends to result in larger particle sizes and non-uniform morphology unless additional milling processes are included.
Hydrothermal/Solvothermal Synthesis
This low-temperature process uses aqueous or organic solvents at high pressure to produce well-crystallized LFP with controlled particle size that is generally in the nanometer range. It provides larger control over morphology and is suitable for high-performance nanostructured LFP powders.
Sol–Gel Method
During this process, metal salts are dissolved to form a gel-like network that ensures molecular-level mixing. Upon calcination and drying, the result is a very homogeneous powder with high purity. The sol-gel process can be utilized to produce fine particles with uniform size distribution, which improves electrochemical performance.
Carbothermal Reduction Method
In this case, carbon serves as a reducing agent and conductive additive. The operation not only produces LFP but also thin carbon coats that enhance electronic conductivity. Carbon source and calcination atmosphere are critical for deciding final performance.
Emerging Techniques
Methods such as microwave-assisted synthesis and spray pyrolysis are being explored to accelerate the reaction, reduce energy consumption, and scale up production while quality remains unaffected. Continuous flow synthesis methods also promise for consistent industrial-scale production.
Further reading: List of Anode and Cathode Battery Materials
Comparison Table: 4 Synthesis Methods
For more differences, please check the table below.
|
Method |
Process Overview |
Typical Particle Size |
Typical Applications |
|
Solid-State Reaction |
Mix Li, Fe, and phosphate precursors (carbonates/oxides /phosphates), then high-temp calcination |
Micrometer scale (1–10 µm) |
General-purpose LFP production where cost > performance |
|
Hydrothermal / Solvothermal |
React precursors in aqueous/organic solvents under high pressure |
Nanometer scale (50–500 nm) |
High-performance nanostructured LFP for fast-charging cells |
|
Sol–Gel |
Dissolve metal salts, form gel, dry and calcine |
Submicron to nanometer scale (50–300 nm) |
Premium-grade LFP for EV and aerospace |
|
Carbothermal Reduction |
Use carbon as reducing agent and conductive additive during calcination |
Submicron to micrometer (0.5–5 µm) |
LFP for high-rate discharge and power tools |
Application of LFP Powder
Lithium-Ion Batteries
LFP is a go-to choice for electric bus fleets in China, where safety and longevity outweigh raw energy density. For instance, many BYD e-buses run on LFP packs that last over 3,000 cycles while maintaining 80% capacity. Its stable 3.2 V output and resistance to thermal runaway make it ideal for high-passenger-load applications.
Stationary Energy Storage
In renewable energy setups, LFP batteries buffer excess solar or wind generation and release it during peak demand. For example, Tesla’s 100 MWh Hornsdale Power Reserve upgrade in Australia incorporates LFP modules for their low maintenance and heat tolerance, operating from −20 °C to 60 °C without active cooling.
Other Uses
LFP is increasingly adopted in microgrids for remote communities, shipboard systems where vibration is constant, and aviation auxiliary power units. Its ability to retain capacity in harsh environments—such as offshore wind farm platforms—makes it a dependable choice where maintenance access is limited.
Conclusion
Lithium Iron Phosphate powder is synonymous with chemical stability, safety, and long cycle life, and it is one of the most indispensable cathode materials for lithium-ion battery applications. Regardless of inferior energy density compared to some alternatives, its durability and cost-effectiveness have provided it with a niche in EVs, stationary storage, and alternative energy technologies. With growing demand for safe and environmentally friendly energy technology across the globe, LFP is going to be a cornerstone material for decades to come.
Reference:
[1] Babkin, Alexander & Kubarkov, Aleksei & Styuf, Elvira & Sergeyev, Vladimir & Drozhzhin, O. & Antipov, Evgeny. (2024). Preparation of battery-grade LiFePO4 by the precipitation method: a review of specific features. Russian Chemical Bulletin. 73. 14-32. 10.1007/s11172-024-4119-8.