Types and Applications of Dopants
What are Dopants?
In materials science, dopants are intentionally added impurities that alter the manner in which a material functions. Even though they might be present in extremely low amounts—sometimes merely a few parts per million—they are capable of drastically altering performance. Doping is most familiar in semiconductor technology, where a tiny variation in carrier type or concentration makes a chip operate as a diode, a transistor, or a solar cell. But the application of dopants extends far beyond electronics. In glasses, polymers, and ceramics, they determine optical properties, mechanical properties, and even chemical stability.
The secret to doping is control: adding the right type and amount of impurity in such a way as to induce desired states or modify those which already exist. Done properly, it enhances conductivity, stabilizes, and tailors a material's response to applied stress. Done poorly, it creates defects or reduced performance. It is for this reason that dopants are among the strongest yet delicate tools in materials science.
 [1]
[1]
Common Types of Dopants
Dopants can be classified according to the effect they produce in the host material. Several broad categories are especially important across science and technology:
1. n-Type Dopants
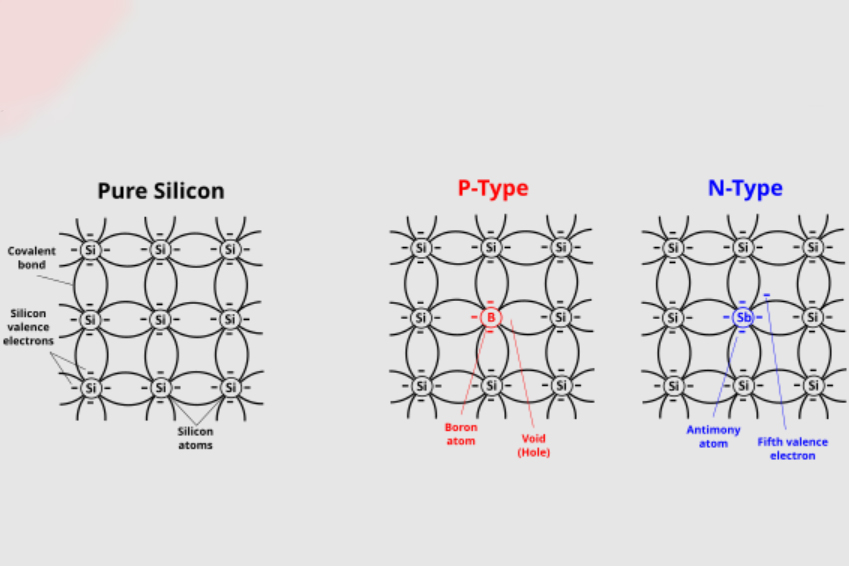
In semiconductors, n-type dopants donate electrons to the conduction band, increasing the number of the negative charge carriers. Silicon doped with phosphorus, arsenic, or antimony is made conductive enough to be utilized as the substrate of integrated circuits. By controlling the levels of dopants, engineers can precisely determine how much current flows in a device and under which conditions.
2. p-Type Dopants
The reverse of n-type doping is p-type doping, in which the impurities create "holes," or positive carriers. Boron, aluminum, or gallium is introduced into silicon so that it has the ability to accept electrons, thus allowing a path for conduction via hole movement. The combination of p-type and n-type regions is employed to make diodes, transistors, and basically all semiconductor logic.
3. Co-Doping
Other sophisticated applications require finer adjustments. Co-doping, or simultaneous addition of two or more dopants, allows fine adjustment of properties. A good example is lithium niobate (LiNbO₃), a compound that is much appreciated for its electro-optic properties. Co-doping with magnesium and zinc imparts lithium niobate optical damage resistance combined with high optical quality. This synergy illustrates how dopants may complement one another rather than operating independently.
4. Optical Dopants
In optics and photonics, rather than regulating current, dopants are used to regulate light. Rare-earth ions such as erbium (Er³⁺), neodymium (Nd³⁺), and ytterbium (Yb³⁺) are doped into crystals, glass, or fibers to create particular absorption and emission lines. Fiber-optic amplifiers, solid-state lasers, and display phosphors all rely on these dopants. The ability of materials to be engineered to absorb and emit light at precise wavelengths has been central to the advancement of modern telecommunications and medical imaging.
5. Ceramic and Glass Dopants
The glass and ceramic systems, too, are highly sensitive to dopants. Substitution with trace amounts of titanium, chromium, or iron can transform color, refractive index, or thermal expansion drastically. Ruby, for instance, is nothing more than chromium-doped aluminum oxide, which creates its celebrated red hue. Titanium doping in alumina, also, increases mechanical strength and resistance to wear to make it even more robust in harsh industrial environments.
6. Polymer Dopants
Conductive polymers are another area of exploration. The majority of polymers are innately insulating such as metals. Doping with materials such as iodine or sulfuric acid can introduce charge carriers by donating or taking electrons away, hence polymers become useful conductors. Doped polymers have opened up new possibilities in flexible electronics, sensors, and energy storage devices.
Summary Table: 6 Common Types of Dopants
|
Dopant Type |
Examples |
Main Effect/Use |
|
n-Type |
Phosphorus, Arsenic, Antimony (in Silicon) |
Donates electrons; increases negative charge carriers; boosts conductivity. |
|
p-Type |
Boron, Aluminum, Gallium (in Silicon) |
Creates “holes” (positive carriers); enables p–n junction devices. |
|
Co-Doping |
Magnesium + Zinc (in LiNbO₃) |
Fine-tunes properties; improves optical damage resistance while keeping performance. |
|
Optical Dopants |
Er³⁺, Nd³⁺, Yb³⁺ (rare-earth ions) |
Enables light absorption/emission; used in lasers, amplifiers, and imaging. |
|
Ceramic/Glass |
Chromium (ruby), Titanium, Iron |
Alters color, strength, refractive index, and thermal/chemical durability. |
|
Polymer Dopants |
Iodine, Sulfuric Acid, Lewis acids |
Introduces charge carriers; makes polymers conductive for electronics and energy use. |
For more information, please check Stanford Electronics.
Key Functions of Dopants
Despite their diversity, dopants perform a few recurring tasks:
- Electrical Conductivity: Dopants control carrier type and concentration in semiconductors and polymers for electronic control.
- Optical Properties: They add discrete energy levels that enable absorption, emission, or nonlinear optics in crystals and glasses.
- Thermal Stability: Certain dopants reduce expansion or stabilize high-temperature forms, enhancing lifetimes of materials.
- Mechanical Enhancement: Dopants toughen ceramics and alloys by influencing grain growth or defect concentration.
- Chemical Resistance: Dopants increase resistance to chemical attack, corrosion, or oxidation of glasses and ceramics.
These uses illustrate how dopants are truly essential in designing next-generation devices and materials.
Several Techniques for Doping
How the dopants are introduced is as important as who they are. Several techniques are used depending on the material and desired effect:
- Diffusion: Dopant atoms are melted along with the host material, with the atoms of the latter diffusing slowly into the lattice. It is a common semiconductor manufacturing method.
- Ion Implantation: Dopant ions are accelerated to high energy and implanted into the host, with precise depth and concentration control. Microelectronics uses it extensively.
- Co-Precipitation or Sol-Gel Processing: The dopants are added during chemical preparation in glasses and ceramics, with uniform dispersal given.
- Melt or Solid-State Reaction: Dopants are introduced prior to crystallization or sintering so that they can become integral to the final material structure.
Every technique has compromises between accuracy, expense, and scalability. Ion implantation provides unmatched control at a high cost, for instance, while melt doping is not as accurate but more suitable for industrial-scale manufacture.
Conclusion
Dopants demonstrate the potential of slight changes. Whether it is electron addition, hole creation, or enabling light emission, they enable materials to do things that were impossible before. From silicon chips and laser crystals to durable ceramics and electrically conducting polymers, dopants have proved to be the behind-the-scenes masters of modern technology.
Reference:
[1] Doping (semiconductor). (2025, August 8). In Wikipedia. https://en.wikipedia.org/wiki/Doping_(semiconductor)