Electric Vehicle Batteries: From Lead-Acid to Lithium-Ion
The development of electric vehicles (EVs) battery has undergone a transformative journey. This article briefs the evolution of electric vehicle batteries, from the early lead-acid batteries to the present-day dominance of lithium-ion technology.
Lead-Acid Batteries: The Early Pioneers
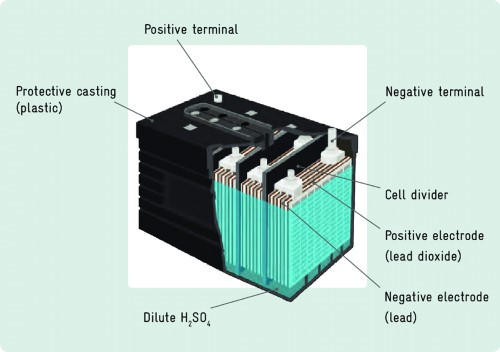
The story of electric vehicle batteries begins in the late 19th century with lead-acid batteries, which were the first widely used energy storage devices in electric vehicles. The lead-acid battery operates through a chemical reaction between lead dioxide (the positive plate), sponge lead (the negative plate), and a sulfuric acid electrolyte, generating electrical energy.
 [1]
[1]
Figure 1. Typical Structure of Lead-Acid Batteries
While lead-acid batteries were revolutionary at the time, they faced several limitations.
- Their energy density was low, meaning they couldn’t store much energy for their size, which resulted in short driving ranges for early electric vehicles.
- Additionally, the lack of widespread charging infrastructure and the slow recharging process made these vehicles impractical for long-distance travel.
Despite these challenges, lead-acid batteries remained in use for decades and continue to be found in applications like automotive starting batteries, uninterruptible power supplies (UPS), and off-grid renewable energy systems.
Nickel-Metal Hydride (NiMH) Batteries: A Step Forward
In the early 20th century, Thomas Edison’s development of the nickel-iron battery offered a glimpse of future possibilities in rechargeable energy storage. Building on this concept, nickel-metal hydride (NiMH) batteries were introduced, offering improved energy density over lead-acid batteries. NiMH batteries use an electrochemical reaction between a positive nickel oxide hydroxide (NiOOH) electrode, a negative metal hydride (MH) electrode, and an alkaline electrolyte.
While NiMH batteries provided a boost in energy storage capacity, they never achieved the widespread adoption seen with lithium-ion technology. Nevertheless, NiMH batteries did play a role in the development of hybrid vehicles, notably in the Toyota Prius, and continue to be used in some applications today.
Lithium-Ion Batteries: The Game Changer
The 21st century marked a turning point in EV battery technology with the widespread adoption of lithium-ion (Li-ion) batteries. These batteries represented a breakthrough in terms of energy density, efficiency, and overall performance, quickly becoming the standard in electric vehicles. Unlike lead-acid or NiMH batteries, lithium-ion batteries utilize lithium ions (Li+) that move between the cathode and anode through a liquid electrolyte, storing and releasing energy during the charge and discharge cycles.
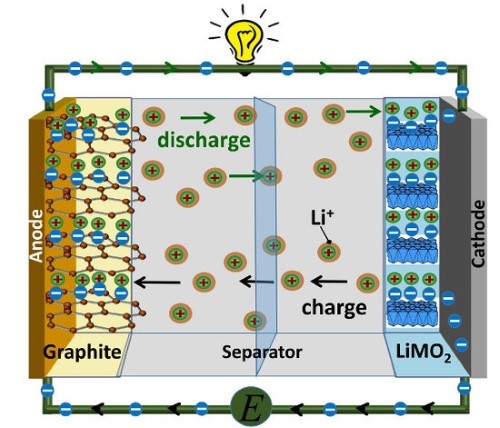 [2]
[2]
Figure 2. Structure of Lithium-Ion Batteries
- What sets lithium-ion batteries apart from previous technologies is their high energy density, meaning they can store more energy in a smaller, lighter package. This is crucial for EVs, as it translates to longer driving ranges without adding significant weight or size to the vehicle.
- Additionally, Li-ion batteries have a low self-discharge rate, meaning they retain their charge for longer periods when not in use, making them more efficient and reliable for long-term use.
- The versatility of lithium-ion technology is another factor that contributed to its success. Different cathode materials, such as lithium cobalt oxide (LiCoO2) for consumer electronics and lithium iron phosphate (LiFePO4) for electric vehicles, allow manufacturers to tailor batteries for specific applications.
The development of lithium nickel cobalt manganese oxide (NCM) and lithium nickel cobalt aluminum oxide (NCA) has further expanded the potential of Li-ion batteries, balancing energy density and power density for use in various applications, from laptops to electric cars.
The Future of EV Batteries
While lithium-ion technology dominates today, the future of EV batteries promises significant advancements in energy storage, safety, and sustainability.
--Solid-State Batteries: A Major Advancement
Solid-state batteries replace the liquid electrolyte in lithium-ion batteries with a solid, offering higher energy density, improved safety, and longer lifespan. These batteries are less prone to fires due to their non-flammable solid electrolyte, and their increased energy density allows for smaller, lighter batteries, boosting EV range.
--Reduced Cobalt in Lithium-Ion Batteries
Reducing cobalt in lithium-ion batteries addresses both environmental and ethical concerns related to cobalt mining. Alternatives like lithium iron phosphate (LiFePO4) are gaining popularity, making battery production more sustainable and reducing costs, making EVs more affordable.
--Fast Charging Technology
Charging time remains a key barrier to EV adoption. Advances in ultra-fast charging, including the use of silicon-based anodes, could drastically cut charging times to minutes.
Conclusion
From the early days of lead-acid batteries to the rise of lithium-ion technology, each step forward has brought greater efficiency, safety, and convenience to electric vehicles. These innovations will help make electric vehicles more accessible, affordable, and sustainable, contributing to a greener future for transportation. For more information, please check Stanford Electronics.
Reference:
[1] Manhart, Andreas & Magalini, Federico & Hinchliffe, Daniel. (2018). End-of-Life Management of Batteries in the Off-Grid Solar Sector How to deal with hazardous battery waste from solar power projects in developing countries? Publication commissioned by: GIZ Sector Project Concepts for Sustainable Solid Waste Management and Circular Economy; developed in collaboration with Energising Development (EnDev).
[2] Madian, M.; Eychmüller, A.; Giebeler, L. Current Advances in TiO2-Based Nanostructure Electrodes for High Performance Lithium Ion Batteries. Batteries 2018, 4, 7. https://doi.org/10.3390/batteries4010007