Fullerenes: The Small Giants in Carbon-Based Electronics
Let’s talk about fullerenes. These carbon molecules may be small, but they’re no lightweight when it comes to materials science. Shaped like tiny cages or tubes, they’ve earned their place in labs and industries alike. And over the years, they’ve proven to be more than just interesting—they’re useful.
 [1]
[1]
What Fullerenes Are, Plain and Simple
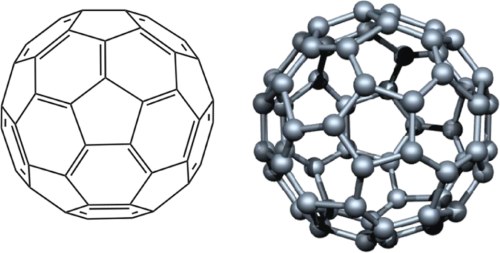
Fullerenes are hollow carbon molecules. Think of them like little soccer balls or tubes, made only from carbon atoms. Some are round like spheres (C60, the most famous), others are stretched like ellipses or columns. They’re related to graphite, but while graphite stacks up in layers of six-membered rings, fullerenes throw in five- and sometimes seven-membered rings. That twist gives them their unique shape and stability.
C60, with 60 carbon atoms, is the one most people talk about. It’s stable, easy to produce now, and has turned out to be very handy in different areas of electronics and physics.
Properties That Matter
Structurally, fullerenes are pretty symmetrical. Always 12 pentagons, and the rest are hexagons. That’s how they stay curved and closed. The smallest one is C20, and as you add more atoms, you get C60, C70, C80, and up.
Now, here’s where it gets interesting. In the early '90s, scientists found that if you added alkali metals to C60—like potassium or cesium—it started acting like a metal. Even more surprising, under the right conditions, these doped fullerenes became superconductors. That means electricity could flow through them with no resistance at low temperatures.
Let’s talk numbers. Potassium-doped C60 showed superconductivity at 18 K. Cesium versions went higher—up to 38 K, though only under pressure. At normal pressure, a compound called Cs2RbC60 holds the record at 33 K. For a molecular solid, that’s impressive.
Why does this happen? It’s got to do with how close the C60 molecules are to each other. Pack them tighter, and the electrons can move around more easily. Loosen them, and the material acts more like an insulator. The balance between how tightly electrons stick to one molecule and how freely they jump around is what drives these changes. We call it a Mott-Hubbard transition. It’s not simple, but the main point is: fullerene solids are tunable. You can make them insulators or conductors, depending on how you handle the atoms around them.
One more thing. The Jahn-Teller effect—yes, a mouthful—means that high-symmetry molecules like C60 can shift a little to save energy. That tiny shift can change how the electrons move, split energy levels, and even help explain why some materials don’t conduct even when they should.
Where Fullerenes Are Used
Capacitors
Fullerenes are finding a real home in energy storage. They’re used in supercapacitors—those devices that charge fast and release power quickly. Fullerenes help by increasing conductivity and improving how much energy the device can hold. Plus, they keep working well after thousands of cycles. This makes them a solid choice in power tools, electric vehicles, and backup energy systems.
Conductive Adhesives
If you build electronics, you know how important a reliable bond is. Fullerenes are used in conductive adhesives to connect chips and components. They help electricity flow while keeping the glue strong. These adhesives also stay flexible, which is useful in things like wearable tech and flexible circuits. They outperform many older materials, especially in high-precision work.
Optoelectronic Devices
Fullerenes shine in optoelectronics. They’re excellent at accepting electrons and help boost performance in devices like organic solar cells (OPVs), photodetectors, and OLEDs. In solar cells, C60 or C70 helps separate electrical charges more efficiently, increasing how much sunlight gets turned into electricity. In OLEDs, they help move electrons across layers, improving brightness and efficiency.
They also work well in organic field-effect transistors (OFETs). These are used in things like display screens, especially where flexibility and lightweight materials matter. Fullerenes keep the devices stable even in less-than-ideal conditions. For more semiconductor materials and applications, please check Stanford Electronics.
FAQs
Q1: Why is C60 the most studied fullerene?
C60 is stable, easy to make in bulk, and has symmetric structure, which makes it predictable and useful in research and applications.
Q2: Are fullerenes used in solar cells today?
Yes. They are key components in some organic photovoltaic (OPV) designs as electron acceptors to improve efficiency.
Q3: What makes fullerene capacitors better than regular ones?
They store more energy, charge faster, and last longer—all thanks to better electron movement and stable molecular structure.
Reference:
[1] Porto, Laís & Silva, Daniela & Oliveira, Ana & Pereira, Arnaldo & Borges, Keyller. (2020). Carbon nanomaterials: synthesis and applications to development of electrochemical sensors in determination of drugs and compounds of clinical interest. Reviews in Analytical Chemistry. 38. 10.1515/revac-2019-0017.
[2] Rama Kanwar Khangarot, Ayushi Bhatnagar, Gangotri Pemawat,
10 - Polymer blend nanocomposites of fullerene for energy storage,
Editor(s): Sabu Thomas, A.R. Ajitha, Maciej Jaroszewski, In Micro and Nano Technologies, Polymer Blend Nanocomposites for Energy Storage Applications, Elsevier, 2023, Pages 293-310, https://www.sciencedirect.com/science/article/pii/B9780323995498000030