Liquid Metal Batteries: Past, Present, and Future
Liquid metal batteries (LMBs) are a compelling energy storage technology, well positioned to address the global demand for large-scale, long-duration energy storage. Elegant and powerful in their design—liquid electrodes and molten salt electrolytes—LMBs offer tremendous advantage in scalability, resilience, and affordability. From their early conceptual start to where they are now, LMBs are a prime candidate for grid-scale energy storage in a world of renewables.
[1]
Introduction to Liquid Metal Batteries
--What Are Liquid Metal Batteries?
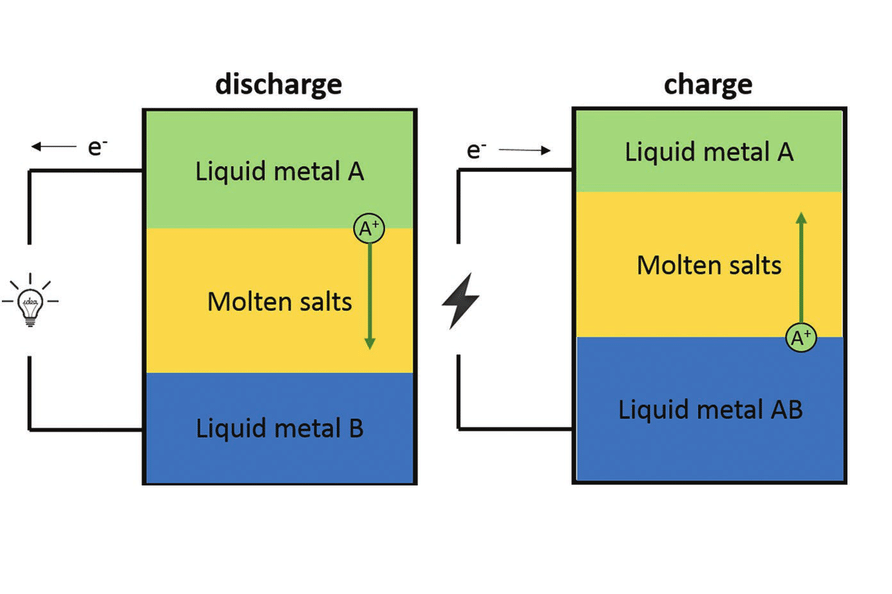
Liquid metal batteries are a rechargeable battery made entirely of molten (liquid) components—two liquid metal electrodes and a molten salt electrolyte. They are designed primarily for grid-scale energy storage, offering a combination of longevity, high efficiency, and low cost well-suited to supporting renewable energy systems.
--Basic Structure and Operation
The most widespread liquid metal battery has three naturally segregated liquids, separated by immiscibility and density:
1. Upper layer (Anode): Low-density liquid metal (e.g., lithium, magnesium, or calcium).
2. Middle layer (Electrolyte): Molten salt that conducts ions but not electrons.
3. Bottom layer (Cathode): High-density liquid metal or metalloid (e.g., antimony, bismuth, or lead).
Because the materials are liquids, they naturally segregate into layers without a separator or membrane.
Further reading: Lithium-Ion Batteries: Function, Materials, and Applications
--How It Works
•During discharge, metal atoms from the anode lose electrons (oxidize) and the resulting ions move from the electrolyte to the cathode.
•On the cathode, these ions receive electrons (reduce) and form alloys or intermetallics.
•In charging, the process is reversed.
--Key Features and Benefits
•High Durability: No solid interfaces lead to minimal structural degradation, resulting in long cycle life.
•Fast Response: Liquid condition enables rapid ion transport, hence appropriate for rapid charge/discharge cycles.
•Low Cost Materials: Generally made with abundant, inexpensive metals, not cobalt or lithium used in common batteries.
•Self-Healing Design: Fluids naturally re-establish proper form after cycling, reducing mechanical stress.
•Thermal Stability: Operates safely at high temperatures (~300–700°C), with internal heat often auto-maintained.
The Development of Liquid Metal Batteries
1. The Past: Origins and Early Development
The evolution of the use of liquid metals in batteries traces back to the 1960s when scientists at Argonne National Laboratory were experimenting with high-temperature electrochemical cells for nuclear energy applications. The first serious model of modern LMBs was the sodium-sulfur (Na-S) battery, which was created in the 1960s by Ford Motor Company and employed in electric vehicles. While not technically a liquid metal battery, it led to molten systems.
The real breakthrough came in the 2000s, when Professor Donald Sadoway and his team at MIT created an actual liquid metal battery architecture. Their architecture included three self-separating liquid layers: a low-density metal (such as magnesium or lithium) as the anode, a high-density metal (such as antimony or bismuth) as the cathode, and a molten salt electrolyte in between. The design bypassed degrading solid interfaces, paving the way for high durability and fast kinetics.
2. The Present: Research, Commercialization, and Challenges
Liquid metal batteries are now progressing from laboratory-scale proof of concept to early commercialization. The front-runner in this class, Ambri founded by Sadoway in 2010, has developed Ca-Sb and Li-Sb systems and is gearing up to deliver grid-scale application batteries with multi-hour storage needs.
Key Advantages:
• High Lifespan: Extensive material degradation by liquid interfaces leads to long cycle life (over 5,000 cycles demonstrated).
• High Power Density: Rapid ion transport in molten media results in rapid charging/discharging.
• Low Cost Materials: Use of earth-abundant metals (Ca, Mg, Sb) reduces cost risks in bulk versus lithium-ion batteries.
• Thermal Efficiency: Operation at 500–700°C allows the battery to harvest heat from resistive losses with little insulation or exterior heating.
Challenges:
• High Operating Temperatures: Maintaining several hundred degrees Celsius limits use in mobile or small-scale systems.
• Sealing and Corrosion: The materials in the containers must withstand harsh conditions for extended durations.
• Manufacturing and Scaling: Manufacturing techniques of such unconventional batteries are still being developed.
New materials (tin, zinc, lead) and room-temperature analogs are researched to expand their use beyond a specific location.
3. The Future: Role in Renewable Energy and Grid Stability
As the world grid transitions to intermittent sources like wind and solar, demand for long-duration cheap and robust storage is skyrocketing. The traditional lithium-ion battery, excellent in transportation and short-duration use, is costly and degrades over the long run on a grid scale.
Liquid metal batteries possess a 10–12 hour discharge window, ideal for grid stabilization in the absence of sunlight or wind. Low maintenance with long lifetimes makes them well suited for microgrids, remote locations, and utility applications.
4. Emerging Trends
•Integration with Renewable Infrastructure: LMBs can be integrated into solar farms or wind parks and store electricity before injecting it into the grid.
•Decentralized Energy Storage: LMBs in smaller, more secure form could power local grids and industrial plants.
•Hybrid Systems: Combination of LMBs with other storage systems such as hydrogen or flow batteries can enable round-the-clock energy optimization.
International research consortia, such as ARPA-E-sponsored consortia, the Horizon programs of the European Union, and Chinese national lab consortia, continue to explore novel chemistries and system designs. If scale-up issues are resolved, storage levelized cost for LMBs could be below $150/kWh, making them very competitive in 2030.
Conclusion
From lab curiosity to possible enabler of the renewable revolution, liquid metal batteries epitomize the peak of energy storage imagination. Their ability to self-segment, corrosion-inert, and high-energy density puts them in prime position to become the backbone of tomorrow's clean but intermittent energy-dominated power grids. With continuing investment in materials science and industrial engineering, liquid metal batteries are set to be the keystone of tomorrow's energy system. For more battery materials at Stanford Electronics, please feel free to contact us.
Reference:
[1] Li, Haomiao & Yin, Huayi & Wang, Kangli & Cheng, Shijie & Jiang, Kai & Sadoway, Donald. (2016). Liquid Metal Electrodes for Energy Storage Batteries. Advanced Energy Materials. 6. 10.1002/aenm.201600483.