Lithium-Ion Batteries: Function, Materials, and Applications
Lithium-Ion Batteries: Function, Materials, and Applications
Introduction
Lithium-ion batteries (LIBs) have revolutionized energy storage, becoming the gold standard for modern technology. From powering smartphones and laptops to enabling electric vehicles and renewable energy storage systems, LIBs have transformed the way we store and use electrical energy.
This article will introduce their fundamental operating principles, key materials, advantages, and applications.
 [1]
[1]
Lithium-Ion Battery Operating Principles
LIBs operate based on the movement of lithium ions (Li+) between the anode and cathode during charge and discharge cycles. During charging, lithium ions move from the cathode to the anode, storing energy. Conversely, during discharge, these ions travel back to the cathode, releasing stored energy to power electronic devices.
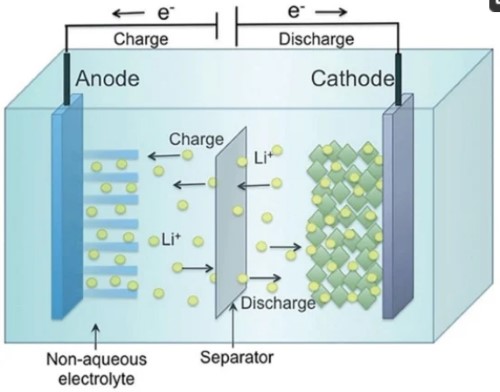 [2]
[2]
Key Materials in Lithium-Ion Batteries
The performance, efficiency, and longevity of LIBs depend on the materials used in their components:
--Anode Materials
The anode stores lithium ions during charging and releases them during discharge. Common anode materials include:
- Graphite: The most widely used anode material due to its stability and reliable electrochemical performance.
- Silicon: Offers higher lithium storage capacity than graphite but faces engineering challenges due to expansion and contraction during charging cycles.
--Cathode Materials
The cathode determines the battery’s voltage and energy capacity. Common cathode materials include:
- Lithium Cobalt Oxide (LiCoO₂): Used in early LIBs, offering high energy density but with cost and safety concerns.
- Lithium Iron Phosphate (LiFePO₄): Known for safety and long cycle life, making it suitable for electric vehicles and renewable energy storage.
- Lithium Nickel Cobalt Manganese Oxide (NCM) & Lithium Nickel Cobalt Aluminum Oxide (NCA): Used in electric vehicles for their balance of energy and power density.
- Lithium Manganese Oxide (LMO): Valued for thermal stability and safety, often used in high-temperature applications.
--Electrolyte
The electrolyte facilitates lithium ion movement between the anode and cathode. Typically composed of lithium salts dissolved in organic solvents, solid-state electrolytes are also being explored for enhanced safety and performance.
--Separator
A porous membrane that prevents short circuits by physically separating the anode and cathode while allowing lithium ions to pass through. Made from materials like polyethylene (PE) or polypropylene (PP), separators are crucial for battery safety.
Advantages of Lithium-Ion Batteries
LIBs are widely used due to their numerous advantages:
- High Energy Density: Offers greater power storage capacity in a compact size.
- Long Cycle Life: Can withstand many charge and discharge cycles before significant degradation.
- Low Self-Discharge Rate: Retains charge efficiently when not in use.
- Safety Features: Modern LIBs include thermal protection, overcharge protection, and built-in battery management systems (BMS) to enhance safety.
- Environmental Benefits: Produces fewer emissions and has a lower environmental footprint compared to traditional energy storage solutions.
Applications of Lithium-Ion Batteries
Due to these advantages, LIBs are widely adopted across various industries:
- Consumer Electronics: Powering smartphones, laptops, tablets, and cameras.
- Electric Vehicles (EVs): Enabling the transition to sustainable transportation by providing efficient and high-capacity energy storage.
- Renewable Energy Storage: Storing electricity from solar and wind power for use during low production periods.
- Aerospace: Used in satellites, spacecraft, and unmanned aerial vehicles (UAVs) for their high energy density and reliability.
- Medical Devices: Essential for powering critical medical equipment such as implantable cardiac defibrillators (ICDs) and portable monitors.
Conclusion
Lithium-ion batteries have transformed multiple industries, offering efficient, durable, and sustainable energy storage solutions. As advancements continue, LIB technology will play a crucial role in shaping the future of energy storage, electric mobility, and renewable energy integration.
A variety of lithium-ion battery materials, including NCM, NCA, LCO, and LFP, are available for purchase at Stanford Electronics. For inquiries, feel free to contact us.
References
- Chandler, D. L. (2023, March 23). Study reveals plunge in lithium-ion battery costs. MIT News. Retrieved September 12, 2023, from MIT News
- Ghiji, M., Novozhilov, V., Moinuddin, K., Joseph, P., Burch, I., Suendermann, B., & Gamble, G. (2020). A Review of Lithium-Ion Battery Fire Suppression. Energies, 13(5117). DOI: 10.3390/en13195117