Essential Electronic Materials: Part 2 - Silicon Carbide
Introduction
Silicon carbide (SiC) is a broadband semiconductor material known for its high hardness, excellent thermal conductivity, and resistance to high temperatures and corrosion. These properties make it a vital material in the electronics industry, especially in power electronics for electric vehicles, 5G communications, photovoltaic power generation, and aerospace. Compared to traditional silicon, SiC offers more efficient energy conversion, lower power consumption, and longer device lifespans, making it a preferred choice for high-performance electronic devices.
Basic Properties of Silicon Carbide
Silicon carbide, with the chemical formula SiC, is synthesized by smelting quartz sand, petroleum coke (or coal coke), and wood chips at high temperatures. It naturally occurs as the rare mineral moissanite but has been mass-produced since 1893. SiC is commonly used as an abrasive, with industrial applications ranging from refractories to semiconductor devices.
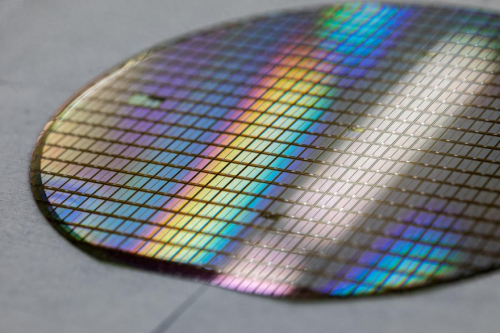
Fig. 1 Silicon Carbide Wafer
There are two main varieties of SiC: black silicon carbide and green silicon carbide. Black SiC contains about 95% SiC and has higher toughness, making it suitable for processing materials like glass, ceramics, and cast iron. Green SiC, with 97% or more SiC content, is self-sharpening and used for precision grinding and processing of harder materials like carbide and titanium alloys. Another variant, cubic silicon carbide (β-SiC), is utilized in ultra-fine machining due to its unique properties.
The SiC crystal structure consists of tetrahedral units, where silicon and carbon atoms are bonded through strong covalent bonds (bond energy of 4.6 eV). The material’s high melting point (approximately 2700 °C) and thermal stability make it suitable for extreme environments. SiC also exhibits a wide range of colors—from light yellow to black—depending on its impurity content.
SiC can exist in over 70 crystalline forms, including the hexagonal or rhombohedral α-SiC and the cubic β-SiC. While α-SiC is the most commonly used form, β-SiC, with its higher surface area, is explored for specific applications like multiphase catalysts.
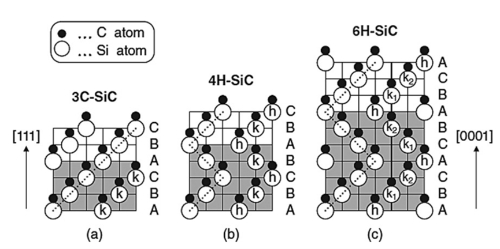
Fig. 2 Crystal structures of major SiC polymorphs
Due to its stable chemical properties, high thermal conductivity, and excellent wear resistance, SiC is used in various applications. For instance, applying SiC powder to turbine impellers or cylinder walls increases their durability and service life. It is also employed in high-grade refractory materials and as a deoxidizer in steelmaking, improving quality and efficiency.
Fabrication Processes
Silicon carbide is primarily produced using two methods: the fusion method and chemical vapor deposition (CVD).
Fusion Method
In the fusion method, silicon and carbon (graphite) are melted together at high temperatures, followed by cooling to form SiC. The process includes:
- Raw Material Preparation: High-purity carbon and silicon materials are crushed and sieved.
- Mixing: The materials are mixed in specific ratios.
- Charging: The mixture is placed in a high-temperature furnace under controlled atmosphere and pressure.
- Carbonization Reaction: The reaction occurs at temperatures between 2000–2500 °C.
- Cooling and Separation: The resulting material is cooled, crushed, and screened to obtain SiC of various particle sizes.
Chemical Vapor Deposition (CVD)
CVD involves depositing silicon and carbon sources in a gas phase to form SiC on a substrate. The process includes:
- Substrate Preparation: Substrates like quartz or graphite are cleaned and treated.
- Reactor Loading: The substrate is placed in a reactor heated to a suitable temperature.
- Reaction Gas Supply: Carbon and silicon-containing gases are introduced at controlled flow rates.
- Gas Phase Reaction: SiC forms through chemical reactions on the substrate surface.
- Cooling and Curing: The reactor is cooled, solidifying the SiC into thin films or blocks.
The choice between these methods depends on the desired properties and applications of the SiC material.
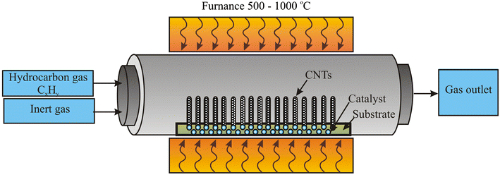
Fig. 3 Facility of Chemical Vapor Deposition (CVD)
Applications of Silicon Carbide
Power Electronics
SiC is transforming power semiconductor devices like MOSFETs and IGBTs. Traditional silicon materials struggle with high currents and voltages, but SiC’s wide-bandgap properties enable low switching losses and energy efficiency, especially in electric vehicles (EVs) and renewable energy systems. For instance, SiC inverters enhance EV range and charging speed while improving efficiency in solar and wind energy systems.
High-Temperature and High-Frequency Devices
SiC’s stability in high-temperature environments makes it ideal for high-frequency applications like 5G base stations and radar systems. Unlike silicon devices, which degrade under high temperatures, SiC maintains performance, supporting high-speed data transmission and efficient operation in communication and military electronics.
LED and Optoelectronic Applications
While gallium nitride has largely replaced SiC in blue LEDs, SiC remains valuable in optoelectronic devices for specific wavelength bands, such as UV and near-infrared. SiC’s radiation-resistant properties make it suitable for extreme optical environments and photodetectors in high-temperature applications.
Fig. 4 Silicon Carbide Wafer for Optoelectronic Applications
Sensors
SiC sensors excel in extreme environments, accurately detecting parameters like gas composition, temperature, and pressure. Their high chemical stability and corrosion resistance make them indispensable in industries like petrochemicals, where traditional sensors fail.
Aerospace and Defense Applications
In aerospace and defense, SiC is used in satellites and missiles due to its high melting point, radiation resistance, and durability. It ensures reliable performance in extreme conditions, enhancing the lifespan and reliability of critical systems like satellite communications and missile control.
Advantages and Limitations of Silicon Carbide
Advantages
- High-Temperature and High-Pressure Performance: SiC remains stable in extreme conditions, offering superior thermal stability and electrical performance compared to silicon.
- High-Frequency and High-Power Applications: SiC’s wide-bandgap characteristics and high carrier mobility reduce energy loss and improve efficiency in high-frequency devices.
- Efficient Energy Conversion: SiC significantly enhances energy efficiency in EVs and renewable energy systems, reducing operational temperatures and improving equipment reliability.

Fig. 5 High-Temperature Silicon Carbide Reduction Tank
Limitations
- High Cost: SiC production and processing are expensive due to the complexity of high-precision processes. The high cost of high-quality SiC crystals impacts its market penetration in cost-sensitive applications.
- Complex Fabrication Process: The slow growth rate and defect-prone nature of SiC crystals result in low yields. Additionally, SiC’s high hardness makes machining challenging.
- Device Reliability: While SiC performs well in extreme conditions, the long-term reliability of some devices remains a challenge. Further improvements are needed to meet the demands of applications requiring extended lifespans.
Conclusion
Silicon carbide (SiC) is a cornerstone material in the field of electronic materials, offering unparalleled advantages in high-temperature, high-frequency, and high-power applications. Its role in power electronics, optoelectronics, sensors, and aerospace underscores its versatility and importance.
Stanford Electronics is a leading supplier of premium silicon carbide materials, delivering dependable solutions to support critical applications across various industries.