Carbon-Based Electronic Materials: Graphene
Introduction to Graphene
Graphene is a rare form of carbon where atoms are contained in one layer in a honeycomb-like structure. Three additional carbon atoms are connected to each carbon atom through sp² hybridization, and a free electron remains in the pz orbital. The structure allows for π-bond formation across the graphene sheet, giving graphene excellent electrical behavior.
A variety of other materials such as carbon nanotubes, quantum dots, and nanoribbons are built upon this basic structure of graphene. When these are piled up in multiple layers (tens of them), they form graphite, where layers are held together by weak van der Waals forces.
The interlayer distance in graphite is around 0.335 nanometers. Because of its unique properties, graphene is considered a revolutionary material, and its applications range from materials science and microelectronics to energy storage and even drug delivery.
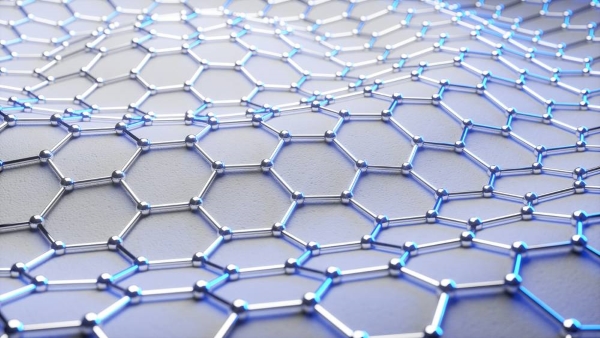
ig 1. Structure of Graphene
Structure and Properties of Graphene
The carbon atoms in graphene are bonded together by sp² hybridized orbitals to form a hexagonal lattice. The uniqueness of the structure of graphene lies in the fact that each carbon atom has four valence electrons. Three of them form bonds with the neighboring carbon atoms, and the fourth one remains in the pz orbital. The three pz orbitals protrude above and below the plane of the graphene, forming π-bonds that extend through the entire layer. The carbon-carbon bond length of graphene is 1.42 × 10⁻¹⁰ meters, and any two neighboring carbon atoms have a bond angle of 120°. This arrangement gives graphene its strength and conductivity.
- Carrier mobility is perhaps one of the key parameters of performance in graphene. Graphene under room temperature conditions has a mobility of about 15,000 cm²/(V-s) and is higher than silicon's by more than ten times. In fact, graphene has a higher mobility than indium antimonide (InSb), the currently known material of highest carrier mobility. At some temperatures, such as at low temperatures, the mobility of graphene can be up to even 250,000 cm²/(V-s). Even more amazing about graphene is that its electron mobility hardly changes with temperature. Even in the temperature range of 50 to 500K, the electron mobility of graphene remains unchanged.
- Graphene possesses some unusual quantum effects as well. The material responds to the half-integer quantum Hall effect, which it achieves when carriers pass through it. It achieves this even under room temperature conditions and because of changes in the chemical potential in the presence of an electric field. Graphene also has a unique feature whereby it does not backscatter electrons if there are impurities, making it superconduct at the local scale. The lack of rest mass in electrons and photons in graphene means that their speed is invariant, regardless of their kinetic energy.
- Also, graphene is a zero-bandgap semiconductor. The conduction and valence bands cross at a point known as the Dirac point, unlike typical semiconductors, which have a broad gap between the two bands. Momentum space in graphene is bifurcated at the Dirac point, unlike typical materials, which are approximately around the Γ point.
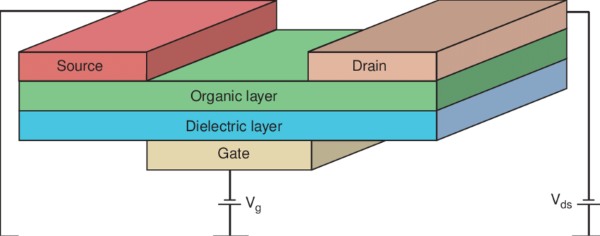
[1]
Fig. 2 The Schematic of A Field Effect Transistor
Applications of Graphene
Graphene has various uses in multiple fields. Its use in electronics, energy storage, and even medical science is being researched and developed enthusiastically.
Integrated Circuits
Graphene is used to make integrated circuits for its outstanding electrical and thermal conductivity. For instance, IBM has developed graphene-based integrated circuits that can be used as broadband RF mixers at frequencies of up to 10 GHz.
Graphene is also used in the fabrication of 3D integrated circuits to address heat dissipation and electromagnetic interference issues. Thus, it is an ideal candidate for future microprocessors and other critical electronic components.
Field Effect Transistors (FETs)
Graphene is an excellent material for field-effect transistors (FETs). Due to its high carrier mobility and atomic thickness, graphene is a perfect material for the channel in such a device.
Graphene FETs are used in RF applications in analog circuits, while in digital circuits, methods like chemical doping can be used to open up the bandgap of graphene. These components come with improved performance and better switching current ratios.
Organic Light Emitting Diodes (OLEDs)
Graphene is known for conductivity and transparency, so it finds uses in OLEDs. Indium tin oxide (ITO) has, in the past, been used in OLEDs, but graphene is increasingly being considered as an alternative to ITO. Graphene electrode-based OLED devices have the same optical and mechanical properties as conventional devices, but with the advantage of flexibility. This opens up the potential for the design of bendable and flexible display devices.
Chemical Sensors
Yet another impressive property is its large specific surface area, so it is quite sensitive to the surrounding environment. Such a property becomes very useful when designing chemical sensors. Sensors made with graphene have been discovered to be very sensitive for the detection of gases such as nitrogen dioxide (NO₂) and ammonia (NH₃). Such sensors are useful in tracking the environment, safety, as well as medical diagnostics.
Optoelectronic Devices
- Graphene is widely used optoelectronics. That’s because of its broad spectral absorption, high conductivity, and flexibility.
- Also, graphene possesses the ultraviolet to infrared wavelength absorbing property, so it is ideal for photodetectors, high-speed fiber optic communications, and terahertz detection.
- Furthermore, graphene's transparency and flexibility could make possible the development of wearable, flexible solar-powered devices.
Conclusion
With tremendous electrical, thermal, and mechanical properties, graphene is ideal for many applications. Anything from integrated circuits and sensors through flexible displays to solar cells may find application for graphene. For more semiconductor materials and applications, please check Stanford Electronics.
Reference:
[1] Reese, Colin & Roberts, Mark & Ling, Mang-mang & Bao, Zhenan. (2004). Organic thin film transistors. Materials Today. 7. 20-27. 10.1016/S1369-7021(04)00398-0.